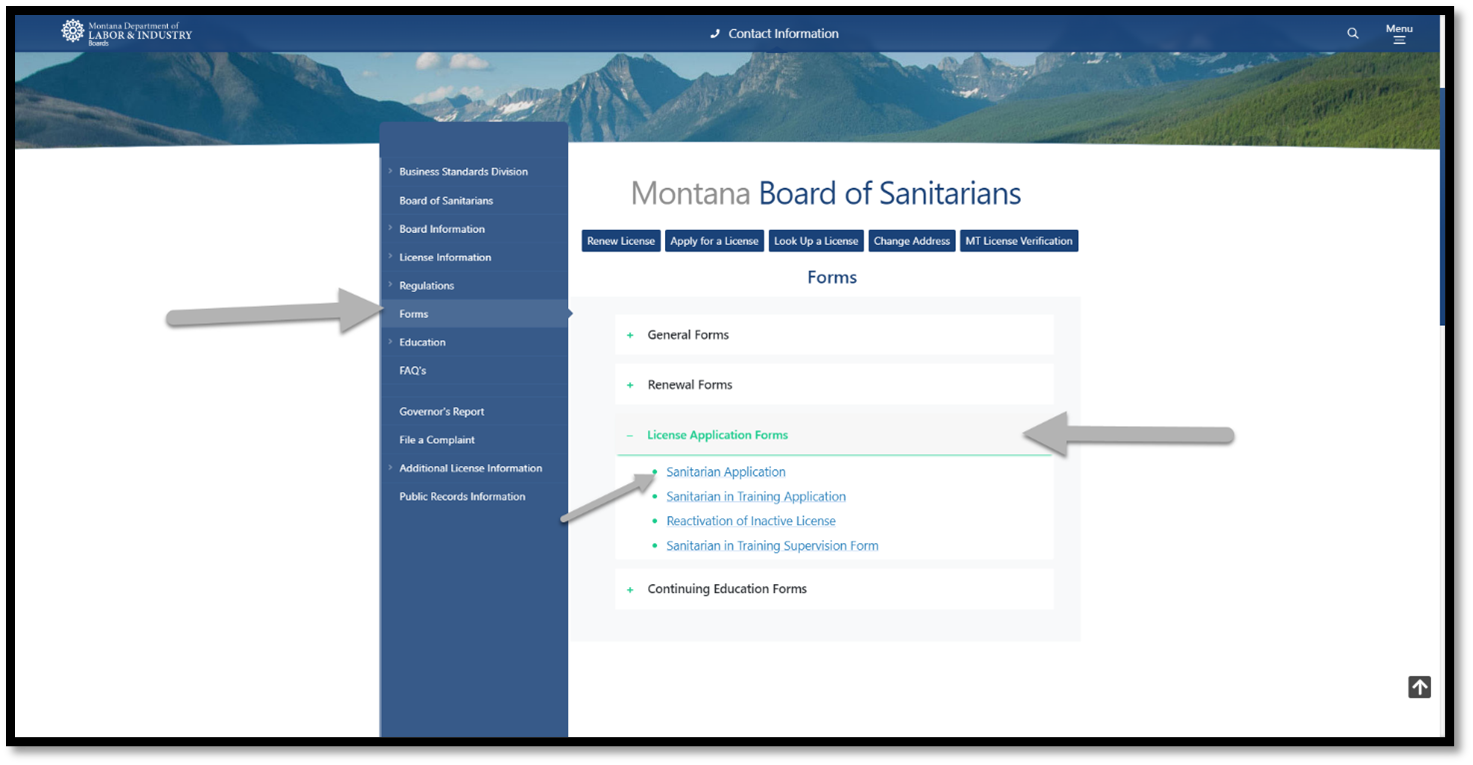
Military Spouse Application Information
The Montana Department of Labor & Industry, Employment Standards Division, is committed to supporting and assisting military members and their spouses obtain professional and occupational licenses. We are dedicated to helping military families work and live in the great State of Montana.
To support professionally licensed military families, please find detailed information about the professional licensing process in the state of Montana and some tips on how to get licensed quickly.